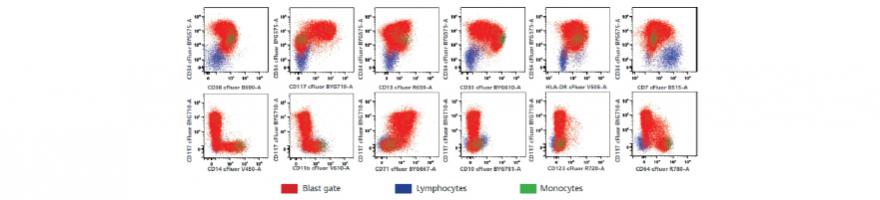
This recent publication in Cytometry Part A describes the development and comprehensive workflow of a single-tube, 19-color assay for identifying and quantifying measurable residual disease (MRD) on an IVD-certified full spectrum flow cytometer. In addition, the authors developed a leukemia-associated immunophenotyping (LAIP) generator to establish a ground truth set of values to validate the semi-automated data analysis and visualization approach they designed for data analysis.
All work was done in accordance with good laboratory practices and conformation recommendations of the European LeukemiaNet Flow-MRD Working Party. The single-tube assay data was validated and compared to data generated on a conventional flow cytometer with a 5-tube assay. Analysis revealed high concordance between the two approaches. However, and importantly, the single-tube semi-automated method revealed an abnormal population that was missed by the conventional 5-tube gating approach. This highlights the importance of a single tube high-dimensional assay and an unbiased, reproducible approach to analysis. A three laser Cytek Northern Lights™-CLC IVD-certified flow cytometer was used to acquire data with the single-tube assay. More detailed information regarding panel design and optimization can be found in the supplemental material.
Read more